Geometric and electronic properties of gold clusters doped with a single oxygen atom†
Abstract
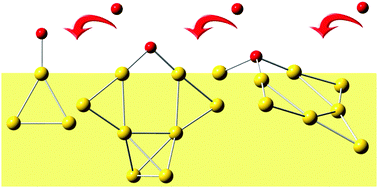
The adsorption behaviour of a single O atom on Aunq clusters (n = 1–8, q = 0, ±1) was systematically investigated by DFT calculations. Both hybrid and pure GGA functionals (B3LYP and PBE) were used to provide reliable conclusions. The most stable structures of AunOq clusters were obtained by using global optimizations with a genetic algorithm. Cationic clusters tend to become three-dimensional for large clusters, as for Au8O+. The binding of O in AunOq clusters is quite strong, especially in the anionic clusters. The O atom can be bound to one, two, or three Au atoms, obtaining nearly one electron from gold atoms. Similarities have been found between AunOq and Aun+1q in terms of geometric structures and binding energies. Frontier molecular orbitals and the distribution of unpaired spin density on the O atom were discussed, both of which have a close relationship with the activity of the clusters.


 Please wait while we load your content...
Please wait while we load your content...