Influence of asymmetric depletion of solvents on the electric double layer of charged objects in binary polar solvent mixtures
Abstract
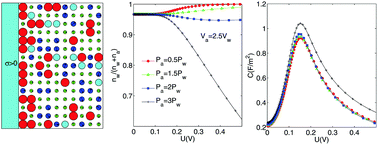
For binary solvent mixtures composed of ions and two kinds of polar solvents, the electric double layer near a charged object is strongly affected by not only the binary solvent composition but also the nature of the solvents, such as the volume and dipole moment of the solvent molecule. Accounting for the difference in sizes of solvents and the orientational ordering of solvent dipoles, we theoretically obtain general expressions for the spatial distribution functions of solvents and ions, in planar geometry and within the mean-field approach. While focusing on long-range electrostatic interaction and neglecting short-range interactions such as preferential solvation, our approach predicts an asymmetric depletion of the two solvents from the charged surface and a behavior of decreased permittivity of the binary solvent mixture. Furthermore, we suggest that the key factor for the depletion is the ratio of the solvent dipole moment to the solvent volume. The influence of the binary solvent composition, the volume of solvent and the dipole moment of the solvent on the number density of solvents, the permittivity and the differential capacitance is presented and discussed. We conclude that accounting for the difference in the volume and dipole moment between polar solvents is necessary for a new approach to represent more realistic situations such as preferential solvation.

 Please wait while we load your content...
Please wait while we load your content...