A new class of N–H excited-state intramolecular proton transfer (ESIPT) molecules bearing localized zwitterionic tautomers†
Abstract
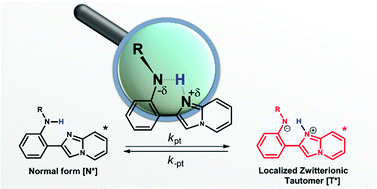
A series of new amino (NH)-type intramolecular hydrogen-bonding (H-bonding) compounds have been strategically designed and synthesized. These molecules comprise a 2-(imidazo[1,2-a]pyridin-2-yl)aniline moiety, in which one of the amino hydrogens was replaced with substituents of different electronic properties. This, together with the versatile capability for modifying the parent moiety, makes feasible comprehensive spectroscopy and dynamics studies of excited-state intramolecular proton transfer (ESIPT) as a function of N–H acidity. Different from other (NH)-type ESIPT systems where the ESIPT rate and exergonicity increase with an increase in the N–H acidity and hence the H-bonding strength, the results reveal an irregular relationship among ESIPT dynamics, thermodynamics and H-bond strength. This discrepancy may be rationalized by the localized zwitterionic nature of 2-(imidazo[1,2-a]pyridin-2-yl)aniline in the proton-transfer tautomer form, which is different from the π-delocalized tautomer form in other (NH)-type ESIPT systems.

 Please wait while we load your content...
Please wait while we load your content...