The effect of halide and iodate anions on the hydrogen-bonding network of water in aqueous nanodrops
Abstract
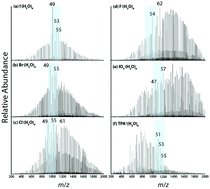
The hydration of halide and iodate anions was investigated using electrospray ionization (ESI) mass spectrometry and infrared photodissociation (IRPD) spectroscopy. The average cluster sizes, determined from the abundances of X−(H2O)n (X− = F−, Cl−, Br−, I−, IO3−) in the ESI mass spectra, follow the order F− > IO3− ≈ Cl− > Br− > I−. The average cluster sizes and solution hydration enthalpies of the halides increase linearly with decreasing ionic radii, but IO3− does not fit this trend. The correlation between average cluster sizes and solution hydration enthalpies indicates that there is a similar relationship between ion–water interactions in these large gas-phase clusters and in bulk solution. The abundances of odd number clusters between n = 49 and 55 for I−, Br− and Cl− are enhanced but those for F− and IO3− are not. I− and IO3− have nearly the same ionic radii, but evidence suggests that these ions interact with water molecules differently both in solution and in small clusters. IRPD spectra of I−(H2O)n and IO3−(H2O)n, measured for select cluster sizes between n = 30 and 75 reveal differences in the hydrogen-bonding network of water molecules in these two ions even for sizes around n = 50. This indicates that differences in hydration motifs reported previously for the first hydration shells of I− and IO3− propagate to water molecules past the second solvation shell, a phenomenon that has not been reported previously for singly charged anions.

 Please wait while we load your content...
Please wait while we load your content...