Impact of the Si/Al ratio on the selective capture of iodine compounds in silver-mordenite: a periodic DFT study†
Abstract
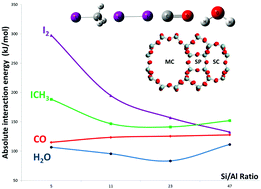
Silver modified zeolites with a mordenite structure can capture volatile iodine compounds (I2 and ICH3) which can be released during a severe nuclear accident. However under these particular conditions, molecules such as CO and H2O present in the containment atmosphere are expected to inhibit the adsorption of iodine compounds. In the present work, periodic density functional theory calculations have been carried out to investigate the interaction of I2, ICH3, H2O and CO molecules in silver-exchanged mordenite with various Si/Al ratios with the aim of finding values that favor a selective adsorption of I2 and ICH3. Computational results show that the interaction energies of CO and H2O remain of the same order of magnitude (from −120 to −140 kJ mol−1 for CO and from −90 to −120 kJ mol−1 for H2O) for all the investigated Si/Al ratios. In contrast, ICH3 is increasingly strongly adsorbed as the Si/Al ratio decreases, from around −145 kJ mol−1 when Si/Al = 47 to −190 kJ mol−1 for Si/Al = 5. The same trend is observed for I2 with a larger amplitude: from −135 kJ mol−1 for Si/Al = 47 to −300 kJ mol−1 for Si/Al = 5. Therefore, the use of silver-exchanged mordenite with Si/Al ratios of 5 or 11 would drastically limit the inhibiting effect of contaminants on the adsorption of volatile iodine species. Also for the same ratios, a spontaneous dissociation of I2 during its adsorption is observed, leading to the formation of AgI complexes which are prerequisite for the immobilization of iodine in the long term.


 Please wait while we load your content...
Please wait while we load your content...