The role of relative rate constants in determining surface state phenomena at semiconductor–liquid interfaces
Abstract
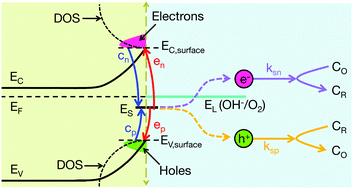
In this work, we present a theoretical study of surface state occupation statistics at semiconductor–liquid interfaces, as it pertains to the evolution of H2 and O2 through water splitting. Our approach combines semiclassical charge transport and electrostatics at the semiconductor–liquid junction, with a master rate equation describing surface state mediated electron/hole transfer. As a model system we have studied the TiO2–water junction in the absence of illumination, where it is shown that surface states might not always equilibrate with the semiconductor. Non-trivial electrostatics, for example including a shifting of the Mott–Schottky plateau in capacitive measurements, are explored when deep-level surface states partially equilibrate with the liquid. We also endeavor to explain observations of non-linearity present in Mott–Schottky plots, as they pertain to surface state occupation statistics. In general, it is intended that the results of this work will serve to further the use and development of quantitative device modeling techniques in the description of H2 evolution at semiconductor–liquid junctions.


 Please wait while we load your content...
Please wait while we load your content...