Comparison of experimental and theoretical quantum-state-selected integral cross-sections for the H2O+ + H2 (D2) reactions in the collision energy range of 0.04–10.00 eV†
Abstract
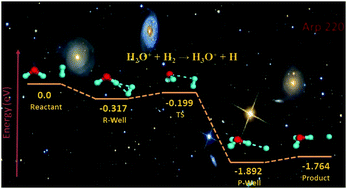
To understand the dynamics of H3O+ formation, we report a combined experimental–theoretical study of the rovibrationally state-selected ion–molecule reactions H2O+(X2B1; v1+v2+v3+; NKa+Kc++) + H2 (D2) → H3O+ (H2DO+) + H (D), where (v1+v2+v3+) = (000), (020), and (100) and NKa+Kc++ = 000, 111, and 211. Both quantum dynamics and quasi-classical trajectory calculations were carried out on an accurate full-dimensional ab initio global potential energy surface, which involves nine degrees of freedom. The theoretical results are in good agreement with experimental measurements of the initial state specific integral cross-sections for the formation of H3O+ (H2DO+) and thus provide valuable insights into the surprising rotational enhancement and vibrational inhibition effects in these prototypical ion–molecule reactions that play a key role in the interstellar generation of OH and H2O species.


 Please wait while we load your content...
Please wait while we load your content...