Phosphine passivated gold clusters: how charge transfer affects electronic structure and stability†
Abstract
A systematic evaluation of small phosphine ligand-protected gold clusters with six to nine gold atoms using density functional theory with dispersion correction has been performed in order to understand the major factors determining stability, including its size, shape, and charge dependence. We show that the charge per atom of the cluster is much more important for the interaction between the ligand shell and gold cluster than the system size. Thus, strong charge transfer effects determine the binding strength between the ligand shell and cluster. The clusters in this series are all non-spherical and exhibit large HOMO–LUMO gaps (above 2.7 eV). Analysis of the delocalized nature of the electronic states at the centre of the clusters demonstrates the presence of nascent superatomic states. However the number of delocalized electrons in these systems is significantly influenced by the charge transfer from the phosphine ligands, contrary to the usual accounting rule for superatom complex systems. Thus, not only electron withdrawing but also charge transfer effects should be considered to influence the superatomic structure of charged ligand surrounded clusters. In consequence in the phosphine gold cluster series under consideration the systems Au7(PPh3)7+ and Au8(PPh3)82+ exhibit nearly fully filled S and P states and the HOMO–LUMO gap increases by 0.2 eV and 0.9 eV, respectively. The interpretation for the stability of the gold phosphine systems is in agreement with experimental results and demonstrates the importance of the superatomic concept.
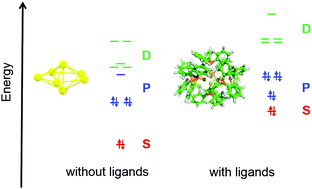

 Please wait while we load your content...
Please wait while we load your content...