Variation of excited-state dynamics in trifluoromethyl functionalized C60 fullerenes†
Abstract
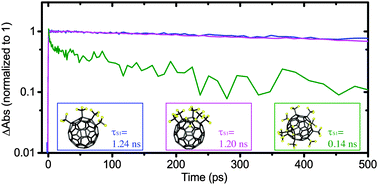
We report on electronically excited-state dynamics of three different trifluoromethyl C60 fullerenes (TMFs, C60(CF3)n: C60/4-1, C60/6-2, and C60/10-1, featuring four, six, and ten trifluoromethyl groups, respectively) using steady-state and time-resolved optical spectroscopy as well as ultrafast pump/probe transient absorption spectroscopy. C60/4-1 and C60/6-2 dissolved in toluene solvent show near-unity S1 → T1 intersystem crossing quantum yield (ΦISC), ca. 1 ns S1-state lifetimes, and microsecond-timescale T1-state lifetimes, which are typical of the fullerene class. On the other hand, C60/10-1 exhibits a dominant sub-nanosecond nonradiative S1 → S0 relaxation mechanism and negligible ΦISC, therefore decreasing the average excited-state lifetime (τavg) by about 5 orders of magnitude compared to that of C60/4-1 and C60/6-2 (τavg ≈ 17 μs and 54 μs for C60/4-1 and C60/6-2, respectively, whereas τavg ≈ 100 ps for C60/10-1). These excited-state characteristics of C60/4-1 and C60/6-2 are preserved in polymer matrix, suggesting that fullerene/polymer interactions do not modulate intrinsic photophysics of trifluoromethyl-substituted fullerenes. The contrasting excited-state study results of C60/4-1 and C60/6-2 to that of C60/10-1 infer that intrinsic optical properties and excited-state dynamics can be affected by the substitution on the fullerene.


 Please wait while we load your content...
Please wait while we load your content...