Calculations of the IR spectra of bend fundamentals of (H2O)n=3,4,5 using the WHBB_2 potential and dipole moment surfaces†
Abstract
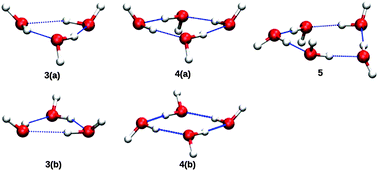
Stimulated by new experiments from the Havenith group, we report IR spectra of the bend fundamentals of (H2O)n=3,4,5, using anharmonic, coupled-mode VSCF/VCI calculations, done in a subspace of modes consisting of all the monomer bends plus the hydrogen-bonded OH stretches. Double-harmonic spectra are also reported. All calculations employ a faster version of the ab initio WHBB potential and also a more accurate representation of the dipole moment surface, reported previously. Comparisons at the harmonic level are made with previous high-level ab initio calculations, notably those of Howard and Tschumper and also with harmonic frequencies from the semi-empirical TTM3-F potential, which have been reported previously by Howard and Tschumper. The calculations provide energies and intensities of the hydrogen-bonded OH stretches and these are also presented and briefly discussed.

 Please wait while we load your content...
Please wait while we load your content...