New solvatochromic probes: performance enhancement via regulation of excited state structures†
Abstract
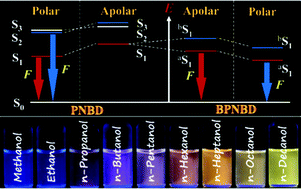
A new fluorescent conjugate (PNBD) with a structure of D–π–A was designed and synthesized, where the donor (D), the acceptor (A) and the bridge (π) are naphthalyl, dicyanovinyl and phenylethynyl–phenylethynyl, respectively. To improve the solubility of the conjugate, two long alkyl chains were introduced as substituents of the central aromatic ring. Spectroscopic studies demonstrated that PNBD is a strongly solvatochromic probe which is characterized by a large molar absorption coefficient (>32 000 cm−1 M−1), long wavelength absorption (>410 nm), large solvatochromic emission range (470–650 nm), high photochemical stability, and good solubility in common organic solvents. The fluorescent quantum yield of PNBD is limited in some polar solvents due to dual emission, a phenomenon ascribed to radiative decay from a higher excited singlet state. To eliminate dual emission, a covalently bound dimer (BPNBD) of PNBD characterized by weak vibronic coupling, was designed and synthesized. The dimer constituents are linked by a single bond between the naphthalyl moieties of the two PNBD monomers. As expected, BPNBD maintains almost all the strong points of the monomer, exhibits a substantial increase in fluorescence quantum yield, and eliminates dual emission by facilitating efficient internal conversion. Importantly, the use of PNBD and BPNBD in concert provides unprecedented discrimination among solvents of similar structures, such as (CH2Cl2, CHCl3, CCl4), (ethyl ether, THF, dioxane), or (methanol, ethanol, n-propanol, n-butanol, n-pentanol, n-hexanol, n-heptanol, n-octanol, n-decanol), allowing rapid and selective visual identification.

 Please wait while we load your content...
Please wait while we load your content...