Brønsted acidity of protic ionic liquids: a modern ab initio valence bond theory perspective†
Abstract
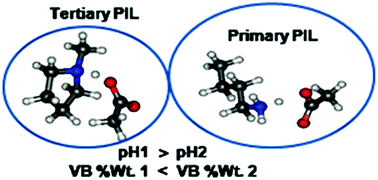
Room temperature ionic liquids (ILs), especially protic ionic liquids (PILs), are used in many areas of the chemical sciences. Ionicity, the extent of proton transfer, is a key parameter which determines many physicochemical properties and in turn the suitability of PILs for various applications. The spectrum of computational chemistry techniques applied to investigate ionic liquids includes classical molecular dynamics, Monte Carlo simulations, ab initio molecular dynamics, Density Functional Theory (DFT), CCSD(t) etc. At the other end of the spectrum is another computational approach: modern ab initio Valence Bond Theory (VBT). VBT differs from molecular orbital theory based methods in the expression of the molecular wave function. The molecular wave function in the valence bond ansatz is expressed as a linear combination of valence bond structures. These structures include covalent and ionic structures explicitly. Modern ab initio valence bond theory calculations of representative primary and tertiary ammonium protic ionic liquids indicate that modern ab initio valence bond theory can be employed to assess the acidity and ionicity of protic ionic liquids a priori.


 Please wait while we load your content...
Please wait while we load your content...