Double imaging photoelectron photoion coincidence sheds new light on the dissociation of energy-selected CH3Cl+ ions†
Abstract
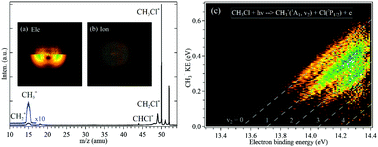
The vacuum ultraviolet (VUV) photoionization and dissociative photoionization of CH3Cl in the energy range of 11–17 eV have been investigated in detail by combining synchrotron radiation and double imaging photoelectron photoion coincidences (i2PEPICO). Three low-lying electronic states of the CH3Cl+ molecular ion, X2E, A2A1 and B2E, were prepared and analyzed. The appearance energies of the energetically accessible fragment ions, CH2Cl+, CHCl+, CH3+ and CH2+, have been obtained from their respective mass-selected threshold photoelectron spectra (TPES) or photoionization efficiency (PIE) curves. The dissociation mechanisms of energy-selected CH3Cl+ ions, prepared in the A2A1 and the B2E electronic states, as well as outside the Franck–Condon region, have been revealed to be state-specific via ion/electron kinetic energy correlation diagrams. In particular, the umbrella mode vibrational progression of the CH3+ fragment ion in the direct dissociation of the A2A1 electronic state was identified and assigned indicating that this state correlates to the CH3+(11A1′) + Cl(2P1/2) dissociation limit, in agreement with the theoretical calculations performed in this work.


 Please wait while we load your content...
Please wait while we load your content...