Water dissociation on MnO(1 × 1)/Ag(100)†
Abstract
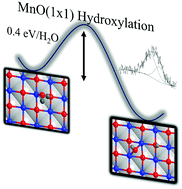
In this work we utilize experimental and simulation techniques to examine the molecular level interaction of water with a MnO(1 × 1) thin film deposited onto Ag(100). The formation of MnO(1 × 1)/Ag(100) was characterized by low energy electron diffraction and scanning tunneling microscopy. Density functional theory (DFT) shows MnO(1 × 1) is thermodynamically more stable than MnO(2 × 1) by ∼0.4 eV per MnO. Upon exposure to 2.5 Torr water vapor at room temperature, X-ray photoemission spectroscopy results show extensive surface hydroxylation attributed to reactivity at MnO(1 × 1) terrace sites. DFT calculations of a water monomer on MnO(1 × 1)/Ag(100) show the dissociated form is energetically more favorable than molecular adsorption, with a hydroxylation activation barrier 0.4 eV per H2O. These results are discussed and contrasted with previous studies of MgO/Ag(100) which show a stark difference in behavior for water dissociation.

 Please wait while we load your content...
Please wait while we load your content...