In situ S-K XANES study of polymer electrolyte fuel cells: changes in the chemical states of sulfonic groups depending on humidity†
Abstract
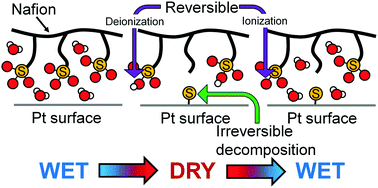
Changes in the chemical states of sulfonic groups of Nafion in polymer electrolyte fuel cells (PEFCs) under gas-flowing conditions were studied using in situ S-K XANES spectroscopy. The applied potential to the electrodes and the humidity of the cell were changed under flowing H2 gas in the anode and He gas in the cathode. While the potential shows no significant effect on the S-K XANES spectra, the humidity is found to induce reversible changes in the spectra. Comparison of the spectral changes with simulations based on the density functional theory calculations indicates that the humidity influences the chemical state of the sulfonic group; under wet conditions the sulfonic group is in the form of a sulfonate ion. By drying treatment the sulfonate ion binds to hydrogen and becomes sulfonic acid. Furthermore, a small fraction of the sulfonic acid irreversibly decomposes to atomic sulfur. The peak energy of the atomic sulfur suggests that the generated atomic sulfur is adsorbed on the Pt catalyst surfaces.

 Please wait while we load your content...
Please wait while we load your content...