Coacervation of α-elastin studied by ultrafast nonlinear infrared spectroscopy†
Abstract
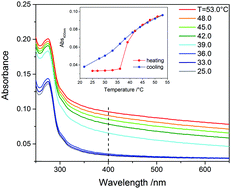
Elastin is the main protein to confer elasticity to biological tissues, through the formation of a hierarchical network of fibres. α-Elastin, a soluble form of the protein, is widely used in studies of the biosynthesis of human elastic tissue and exhibits coacervation in solution. This process involves the association of α-elastin molecules through a liquid–liquid phase transition, which is reversible unless the temperature is driven sufficiently high to induce the formation of insoluble aggregates. The thermodynamics of this process have attracted interest over many years and in the present work we used ultrafast nonlinear infrared spectroscopy of the amide I protein backbone vibration to resolve the secondary structural changes occurring during coacervation and probe the protein dynamics on a picosecond time scale. Four classes of carbonyl oscillators with distinct absorption peaks were revealed and, through narrowband excitation, vibrational and anisotropy decays could be distinguished. Analysis of the vibrational lifetimes and anisotropy decay times of these bands characterized the conformational changes and revealed the structural bases of the coacervation process.


 Please wait while we load your content...
Please wait while we load your content...