PCl3–C6H6 heterodimers: evidence for P⋯π phosphorus bonding at low temperatures†
Abstract
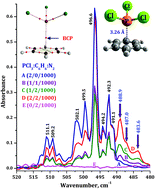
A phosphorous trichloride (PCl3)–benzene (C6H6) heterodimer was generated in a low temperature N2 matrix and was characterized using infrared spectroscopy. The structure of the heterodimer produced in the matrix isolation experiment was discerned through ab initio computations. Computations disclosed that the experimentally detected dimer is stabilized through strong non-covalent phosphorus bonded P⋯π interaction, considered as a class of pnicogen bonding. This experimentally unmapped P⋯π interaction so far has been reconnoitered using atoms in molecules and natural bond orbital and energy decomposition analyses. The influence of substitutions on both the PCl3 and C6H6 monomeric units of the heterodimer was subsequently examined to understand the strength of P⋯π interaction as a result of these substitutions.

 Please wait while we load your content...
Please wait while we load your content...