The divide-and-conquer second-order proton propagator method based on nuclear orbital plus molecular orbital theory for the efficient computation of proton binding energies†
Abstract
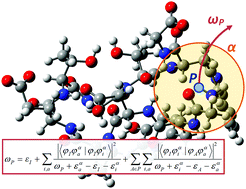
An efficient computational method to evaluate the binding energies of many protons in large systems was developed. Proton binding energy is calculated as a corrected nuclear orbital energy using the second-order proton propagator method, which is based on nuclear orbital plus molecular orbital theory. In the present scheme, the divide-and-conquer technique was applied to utilize local molecular orbitals. This use relies on the locality of electronic relaxation after deprotonation and the electron–nucleus correlation. Numerical assessment showed reduction in computational cost without the loss of accuracy. An initial application to model a protein resulted in reasonable binding energies that were in accordance with the electrostatic environment and solvent effects.

 Please wait while we load your content...
Please wait while we load your content...