The Ebola virus protein VP40 hexamer enhances the clustering of PI(4,5)P2 lipids in the plasma membrane†
Abstract
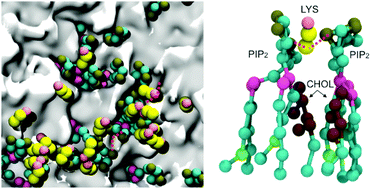
The Ebola virus is a lipid-enveloped virus that obtains its lipid coat from the plasma membrane of the host cell it infects during the budding process. The Ebola virus protein VP40 localizes to the inner leaflet of the plasma membrane and forms the viral matrix, which provides the major structure for the Ebola virus particles. VP40 is initially a dimer that rearranges to a hexameric structure that mediates budding. VP40 hexamers and larger filaments have been shown to be stabilized by PI(4,5)P2 in the plasma membrane inner leaflet. Reduction in the plasma membrane levels of PI(4,5)P2 significantly reduce formation of VP40 oligomers and virus-like particles. We investigated the lipid–protein interactions in VP40 hexamers at the plasma membrane. We quantified lipid–lipid self-clustering by calculating the fractional interaction matrix and found that the VP40 hexamer significantly enhances the PI(4,5)P2 clustering. The radial pair distribution functions suggest a strong interaction between PI(4,5)P2 and the VP40 hexamer. The cationic Lys side chains are found to mediate the PIP2 clustering around the protein, with cholesterol filling the space between the interacting PIP2 molecules. These computational studies support recent experimental data and provide new insights into the mechanisms by which VP40 assembles at the plasma membrane inner leaflet, alters membrane curvature, and forms new virus-like particles.


 Please wait while we load your content...
Please wait while we load your content...