NX⋯Y halogen bonds. Comparison with NH⋯Y H-bonds and CX⋯Y halogen bonds†
Abstract
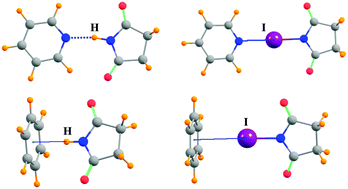
Quantum calculations examine how the NH⋯Y H-bond compares to the equivalent NX⋯Y halogen bond, as well as to comparable CH/CX donors. Succinimide and saccharin, and their corresponding halogen-substituted derivatives, are chosen as the prototype NH/NX donors, paired with a wide range of electron donor molecules. The NH⋯Y H-bond is weakened if the bridging H is replaced by Cl, and strengthened by I; a Br halogen bond is roughly comparable to a H-bond. The lone pairs of the partner molecule are stronger electron donors than are π-systems. Whereas Coulombic forces represent the largest fraction of the attractive force in the H-bonds, induction energy is magnified in the halogen bonds, surpassing electrostatics in several cases. Mutation of NH/NX to CH/CX weakens the binding energy to roughly half its original value, while also lengthening the intermolecular distances by 0.3–0.8 Å.

 Please wait while we load your content...
Please wait while we load your content...