Tautomerization lowers the activation barriers for N-glycosidic bond cleavage of protonated uridine and 2′-deoxyuridine†
Abstract
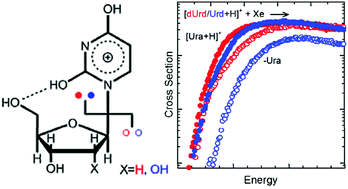
The gas-phase conformations of protonated uridine, [Urd+H]+, and its 2′-deoxy form, protonated 2′-deoxyuridine, [dUrd+H]+, have been examined in detail previously by infrared multiple photon dissociation action spectroscopy techniques. Both 2,4-dihydroxy tautomers and O4 protonated conformers of [Urd+H]+ and [dUrd+H]+ were found to coexist in the experiments with the 2,4-dihydroxy tautomers dominating the population. In the present study, the kinetic energy dependence of the collision-induced dissociation behavior of [Urd+H]+ and [dUrd+H]+ are examined using a guided ion beam tandem mass spectrometer to probe the mechanisms and energetics for activated dissociation of these protonated nucleosides. The primary dissociation pathways observed involve N-glycosidic bond cleavage leading to competitive elimination of protonated or neutral uracil. The potential energy surfaces (PESs) for these N-glycosidic bond cleavage pathways are mapped out via electronic structure calculations for the mixture of 2,4-dihydroxy tautomers and O4 protonated conformers of [Urd+H]+ and [dUrd+H]+ populated in the experiments. The calculated activation energies (AEs) and heats of reaction (ΔHrxns) for N-glycosidic bond cleavage at both the B3LYP and MP2(full) levels of theory are compared to the measured values. The agreement between experiment and theory indicates that B3LYP provides better estimates of the energetics of the species along the PESs for N-glycosidic bond cleavage than MP2, and that the 2,4-dihydroxy tautomers, which are stabilized by strong hydrogen-bonding interactions, predominantly influence the observed threshold dissociation behavior of [Urd+H]+ and [dUrd+H]+.


 Please wait while we load your content...
Please wait while we load your content...