Alcohols as molecular probes in ionic liquids: evidence for nanostructuration†
Abstract
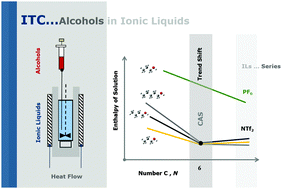
A comprehensive study of the solution and solvation of linear alcohols (propan-1-ol, butan-1-ol and pentan-1-ol) in ionic liquids (ILs) is presented. The effect of the alkyl chain size of both alcohols and ILs (1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, [CnC1im][NTf2], ionic liquid series) on the thermodynamic properties of solution and solvation was used to obtain insight into the interactions between alcohols and ILs. Alcohols were used as molecular probes to ascertain whether their solvation in ILs would reflect IL nanostructuration. A trend shift was found in the values of enthalpy of solution and solvation for the [CnC1im][NTf2] series at a critical alkyl size (CAS) of C6. Further, the effect of the hydrogen bond basicity of the anion in the solvation of alcohols was explored based on the comparative study of the solvation of propan-1-ol in two different IL series, 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [CnC1im][NTf2] and hexafluorophosphate [CnC1im][PF6]. The results obtained provide experimental support for the strength of hydrogen bonds between the alcohols and the NTf2 and PF6 anions, providing insights into the IL intermolecular interactions, namely by indicating the ability of the alcohols to discriminate the IL anion hydrogen bond basicity.


 Please wait while we load your content...
Please wait while we load your content...