Anion replacement at Au(110)/electrolyte interfaces
Abstract
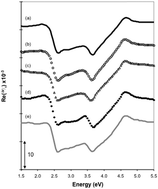
A characteristic reflection anisotropy spectrum (RAS) is observed from a Au(110) surface in a wide range of electrolytes and combinations of pH and applied potentials. It is suggested that this common RAS profile arises from an interaction between the potential applied to the Au(110) electrode and the dipole moments of oxidized species that locates the Fermi level at a common position with respect to the electronic band structure of Au. Rapid changes in this RAS profile are observed for Au(110)/H2SO4 as the potential is switched between 0.3 V and 0.6 V, a potential range in which the surface is not reconstructed and below the potential range of surface oxidation. The spectral changes are completed in less than 10 ms, are reversible and are attributed to the replacement of adsorbed anions by an oxygenated species.

 Please wait while we load your content...
Please wait while we load your content...