Acidity constants and redox potentials of uranyl ions in hydrothermal solutions
Abstract
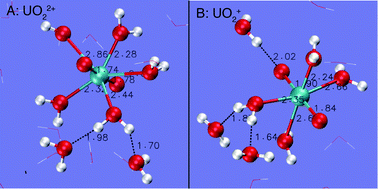
We report a first principles molecular dynamics (FPMD) study of the structures, acidity constants (pKa) and redox potentials (E0) of uranyl (UO22+) from ambient conditions to 573 K. It is found that UO22+ keeps five coordination up to 573 K whereas UO2+ transforms from 5 to 4-coordinate as temperature increases to 573 K. The FPMD-based vertical energy gap method is used to derive pKas and E0s. The method is validated by comparing with available experimental data (for E0 under the ambient conditions and for pKas from ambient conditions to 367 K), with an uncertainty of 1–2 pKa units and 0.2 V for pKa and E0. The encouraging results demonstrate that the method may be used to predict the pH–Eh diagrams of f-block elements under the conditions of hydrothermal solutions. The results show that the acidity constants of uranyl decrease with temperature and are lower than 3.0 when the temperature is above 473 K, indicating that hydrolytic forms are dominant for U(VI) in the near neutral pH range. The reduction potential increases with temperature, indicating that the reduced state is more significant at higher temperatures.

 Please wait while we load your content...
Please wait while we load your content...