Photoelectron spectroscopic and computational study of the PtMgH3,5− cluster anions†
Abstract
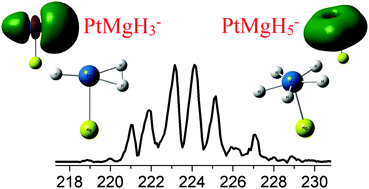
The two cluster anions, PtMgH3− and PtMgH5−, were studied by photoelectron spectroscopy and theoretical calculations. Experimentally-determined electron affinity (EA) and vertical detachment energy (VDE) values were compared with those predicted by our computations; excellent agreement was found. The calculated structures of PtMgH3− and PtMgH3 both exhibit η2-bonded H2 moieties. Activation of these H2 moieties is implied by the elongation of their bond lengths relative to the bond length of free H2. The calculated structures of PtMgH5− and PtMgH5 both exhibit all-hydrogen, five-member, σ-aromatic rings. These attributes are responsible for this anion's special stability.

 Please wait while we load your content...
Please wait while we load your content...