Structural and dynamic evolution of the amphipathic N-terminus diversifies enzyme thermostability in the glycoside hydrolase family 12†
Abstract
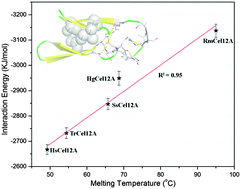
Understanding the molecular mechanism underlying protein thermostability is central to the process of efficiently engineering thermostable cellulases, which can provide potential advantages in accelerating the conversion of biomass into clean biofuels. Here, we explored the general factors that diversify enzyme thermostability in the glycoside hydrolase family 12 (GH12) using comparative molecular dynamics (MD) simulations coupled to a bioinformatics approach. The results indicated that protein stability is not equally distributed over the whole structure: the N-terminus is the most thermal-sensitive region of the enzymes with a β-sandwich architecture and it tends to lose its secondary structure during the course of protein unfolding. Furthermore, we found that the total interaction energy within the N-terminus is appreciably correlated with enzyme thermostability. Interestingly, the internal interactions within the N-terminus are organized in a special amphipathic pattern in which a hydrophobic packing cluster and a hydrogen bonding cluster lie at the two ends of the N-terminus. Finally, bioinformatics analysis demonstrated that the amphipathic pattern is highly conserved in GH12 and besides that, the evolution of the amino acids in the N-terminal region is an inherent mechanism underlying the diversity of enzyme thermostability. Taken together, our results demonstrate that the N-terminus is generally the structure that determines enzyme thermostability in GH12, and thereby it is also an ideal engineering target. The dynameomics study of a protein family can give a general view of protein functions, which will offer a wide range of applications in future protein engineering.

 Please wait while we load your content...
Please wait while we load your content...