Rate constant calculations of the GeH4 + OH/OD → GeH3 + H2O/HOD reactions using an ab initio based full-dimensional potential energy surface†
Abstract
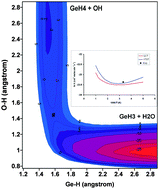
We report an analytical full-dimensional potential energy surface for the GeH4 + OH → GeH3 + H2O reaction based on ab initio calculations. It is a practically barrierless reaction with very high exothermicity and the presence of intermediate complexes in the entrance and exit channels, reproducing the experimental evidence. Using this surface, thermal rate constants for the GeH4 + OH/OD isotopic reactions were calculated using two approaches: variational transition state theory (VTST) and quasi-classical trajectory (QCT) calculations, in the temperature range 200–1000 K, and results were compared with the only experimental data at 298 K. Both methods showed similar values over the whole temperature range, with differences less than 30%; and the experimental data was reproduced at 298 K, with negative temperature dependence below 300 K, which is associated with the presence of an intermediate complex in the entrance channel. However, while the QCT approach reproduced the experimental kinetic isotope effect, the VTST approach underestimated it. We suggest that this difference is associated with the harmonic approximation used in the treatment of vibrational frequencies.

 Please wait while we load your content...
Please wait while we load your content...