A multi-technique comparison of the electronic properties of pristine and nitrogen-doped polycrystalline SnO2†
Abstract
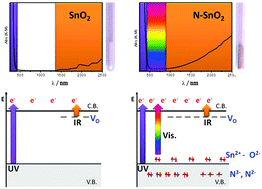
Nitrogen doped tin(IV) oxide (SnO2) materials in the form of nanometric powders have been prepared by precipitation with ammonia. Their properties have been compared with those of undoped materials obtained in a similar way using various physical techniques such as photoelectron spectroscopies (XPS and UPS), UV-Vis-NIR spectroscopy and electron paramagnetic resonance (EPR). Nitrogen doping leads to the formation of various nitrogen containing species, the more relevant of which is a nitride-type ionic species, based on the substitution of a lattice oxygen atom with a nitrogen atom. This species exists in two forms, paramagnetic (hole centre, formally N2−) and diamagnetic (N3−). The mutual ratio of the two species varies according to the oxidation state of the material. The doped solid, like most of the semiconducting oxides, tends to lose oxygen forming oxygen vacancies upon annealing under vacuum and leaving an excess of electrons in the solid. The stoichiometry of the solid can thus be markedly changed depending on the external conditions. Excess electrons are present both as itinerant electrons in the conduction band and as Sn(II) states lying close to the valence band maximum. The presence of nitride-type centres, which are low energy states located below the top of the valence band, decreases the energy cost for the formation of oxygen vacancies by O2 release from the lattice. This particular feature of the doped system represents a severe limit to the preparation of a p-type SnO2via nitrogen doping.

 Please wait while we load your content...
Please wait while we load your content...