Weak C–H acids as protonophores can carry hydrogen ions through lipid membranes and mitochondria: a case of o-carborane
Abstract
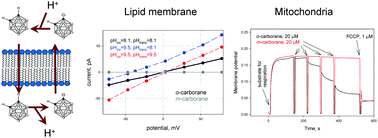
ortho-Carborane (1,2-C2B10H12) was found to be a carrier of protons in both mitochondrial and artificial lipid membranes, suggesting that this dicarborane can reversibly release hydrogen ions and diffuse through the membranes in neutral and anionic forms. Similar to conventional uncouplers (e.g. 2,4-dinitrophenol), o-carborane stimulated mitochondrial respiration and decreased the membrane potential at concentrations of tens of micromoles. Protonophoric activity of o-carborane was observed both by a fluorometric assay using pyranine-loaded liposomes and electrical current measurements across planar lipid bilayers. Substantial contribution of the proton flux to the o-carborane-mediated current was proved by a shift of the zero current voltage upon imposing a pH gradient across the membrane. Meta-carborane (1,7-C2B10H12) lacked the protonophoric activity in line with its reduced C–H acidity. The results suggest that weak C–H acids can exhibit protonophoric activity in the biological environment. The finding of a new class of protonophoric compounds is of substantial interest due to promising anti-obesity and anti-diabetic properties of uncouplers.

 Please wait while we load your content...
Please wait while we load your content...