Probing the spontaneous reduction mechanism of platinum ions confined in the nanospace by X-ray absorption fine structure spectroscopy
Abstract
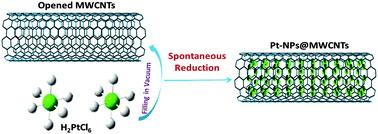
The reduction mechanism of Pt4+ ions confined in the channel of multi-walled carbon nanotubes was mainly investigated using X-ray absorption fine structure (XAFS) spectroscopy, with the aid of TEM, Raman, XRD and ICP-AES studies. The XAFS spectra revealed the spontaneous formation of Pt nanoparticles when H2PtCl6 was confined in multi-walled carbon nanotubes (MWCNTs). The Pt L3-edge X-ray absorption near edge structure (XANES) coupled with the C K-edge NEXAFS results indicated that the reduction of Pt4+ from tetravalent to zerovalent was attributed to the electron transfer from MWCNTs. The Fourier transform R-space of the Pt L3-edge XAFS data displayed that the nanoconfinement effect of MWCNTs promoted the formation of Pt nanoparticles. Moreover, the Pt–Pt bond length in confined Pt nanoparticles became shorter than that of Pt in the bulk state. Furthermore, by varying the inner diameter of MWCNTs from 15 nm to 10 nm and 5 nm, the Pt–Pt bond length of nanoconfined Pt nanoparticles decreased gradually. The results clearly revealed that MWCNTs acting as enriched electron donors can continuously reduce the confined Pt ions to Pt nanoparticles, thereby showing a great potential for the design of a new type of confined nanocatalysts.

 Please wait while we load your content...
Please wait while we load your content...