Sign inversion of surface stress–charge response of bulk nanoporous nickel actuators with different surface states†
Abstract
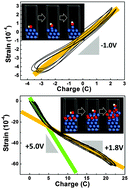
The surface stress–charge coefficient, ζ, is a fundamental material parameter and reflects the response of surface stress to the change of superficial charge. The sign and the quantity of ζ play a crucial role in electrochemically induced actuation of nanostructured metals. Here, for the first time, we address the electrochemical actuation and the associated stress–charge coefficients of bulk nanoporous nickel (np-Ni) in both strongly (NaOH) and weakly (NaF) adsorbed electrolytes. The results reveal a normal negative value of ζ for the np-Ni with the clean surface, and unusual positive values of ζ for the oxide-covered surface. Interestingly, the oxidized np-Ni cannot recover the conventional negative value of ζ even in the cathodic potential window. Moreover, the reversible strain amplitude and the involved charge are quite different in distinct potential windows (the same electrolyte) or in different electrolytes (strongly or weakly adsorbed). In addition, density functional theory (DFT) calculations have been performed to understand the electrochemical actuation behaviors of the np-Ni with different surface states. In some aspects, the scenario of the np-Ni indeed differs from that of nanoporous noble metals like Au or Pt. Our findings provide useful information on understanding the electrochemical actuation of nanostructured metals, and novel actuators or sensors could be developed based upon earth-abundant metals like Ni, Co, and so forth.

 Please wait while we load your content...
Please wait while we load your content...