Adsorption of indole and quinoline from a model fuel on functionalized MIL-101: effects of H-bonding and coordination
Abstract
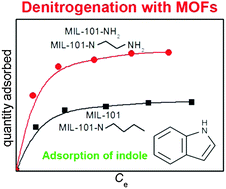
Denitrogenation of a model fuel was studied by employing the adsorption of indole (IND) and quinoline (QUI) over a metal–organic framework (MOF), MIL-101, with or without functionalization. Five MIL-101 MOFs were obtained by direct syntheses, grafting, and hydrogenation. The adsorption capacity of IND increased significantly (up to 1.7 times that of MIL-101) upon introducing amino functional groups into MIL-101, despite the decrease in the porosity of the MOF after modification. However, the adsorption of QUI decreased when MIL-101 was modified using both amino and butyl groups because of the reduced porosity. The adsorption capacity for IND (based on the unit surface area of MIL-101s) showed that MIL-101s with amino groups had an adsorption capacity of around 2.3 times those of MIL-101 or MIL-101 with butyl groups, showing the importance of H-bonds for the adsorption of IND over MIL-101s. However, for the adsorption of QUI, only the porosity is important, and coordination of QUI on open metal sites does not play a dominant role, probably because of the low basicity of QUI. Moreover, there is little contribution of H-bonds (between N of QUI and H of –NH2 of MOF) in the adsorption of QUI over amino-MIL-101s, and this is probably due to a similar reason. Preparation methods for MIL-101 having a free amino group did not have any effect on the adsorption (based on surface area) of QUI or IND.

 Please wait while we load your content...
Please wait while we load your content...