Gold nanoparticles in aqueous solutions: influence of size and pH on hydrogen dissociative adsorption and Au(iii) ion reduction†
Abstract
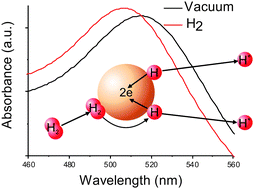
The shift of the localized surface plasmon resonance (LSPR) band of gold nanoparticles to shorter wavelengths upon saturation of the hydrosol with hydrogen is used as a tool to study the electrochemical processes on the particle surface. It is shown that dissociative adsorption of hydrogen takes place on the surface of a particle and results in the migration of a proton into the dispersion medium, while the electron remains on the nanoparticle, i.e., a hydrogen-like nanoelectrode is formed. It is shown that Au(III) ions can be reduced on the gold nanoelectrodes. A thermodynamic scheme explaining the shift of the LSPR band is used to explain the peculiarities of the Au(III) ion reduction. The reduction rate does not depend on the ion concentration and varies linearly with pH. The observed correlations are explained in terms of a simple model of electrochemical processes taking place on the nanoparticle as an electrode. It is shown that with an increase in the particle size, its capacity for dissociative adsorption of hydrogen decreases and the Au(III) reduction slows down.


 Please wait while we load your content...
Please wait while we load your content...