Variation in the c-axis conductivity of multi-layer graphene due to H2 exposure
Abstract
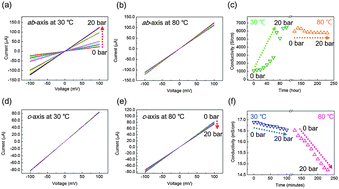
The variation of the c-axis conductivity of a multilayer graphene (MLG) as a function of H2 pressure from vacuum to 20 bar has been investigated. MLG was connected to the electrodes vertically using a wet transfer process. After exposure to H2 gas pressure up to 20 bar, the chemisorption of dissociated atomic hydrogen on MLG affects its electrical and structural properties. The formation of C–H bonds causes a decoupling of graphene layers, and then interferes with charge transfer through the out of plane. As a result, the c-axis conductivity decreases. Furthermore, the electron doping effect and the decoupling of the layers were confirmed using Raman spectroscopy. Hydrogenated carbons induce a defect structure of MLG which results in the expansion of layers. We observed a 43.54% increase in the thickness of the MLG after H2 exposure using atomic force microscopy.

 Please wait while we load your content...
Please wait while we load your content...