Absence of a stable atomic structure in fluorinated graphene
Abstract
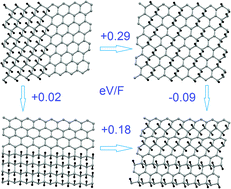
Based on the results of first-principles calculations we demonstrate that significant distortion of graphene sheets caused by adsorption of fluorine atoms leads to the formation of metastable patterns for which the next step of fluorination is considerably less energetically favorable. Existence of these stable patterns oriented along the armchair direction makes possible the synthesis of various CFx structures. The combination of strong distortion of the nonfluorinated graphene sheet with the doping caused by the polar nature of C–F bonds reduces the energy cost of migration and the energy of migration barriers, making possible the migration of fluorine atoms on the graphene surface as well as transformation of the shapes of fluorinated areas. The decreasing energy cost of migration with increasing fluorine content also leads to increasing numbers of single fluorine adatoms, which could be the source of magnetic moments.

 Please wait while we load your content...
Please wait while we load your content...