Amplified fluorescence emission of bolaamphiphilic perylene-azacrown ether derivatives directed towards molecular recognition events†
Abstract
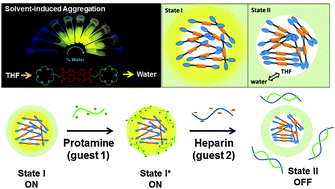
Long-term creative approaches have been considered in the design of molecular probes to overcome the quenching effect of important dyes in an aqueous medium. Using the rational donor–acceptor based design principle, we demonstrate herein the different fluorescence states of a non-conjugated symmetrical perylene-azacrown ether system in a solution, from the molecular to the aggregated states. The ethylene-spacer is exceptionally capable of fluorescence enhancement, even in the aggregated state (organic nanoparticle, ONPs, 44 nm), overcoming the quenching effect on changing the solvent from tetrahydrofuran to water. The ONPs with crown ether receptors at the surface show colloidal stability in an aqueous solution. Furthermore, an improved fluorescent state is developed via ONPs–polymer (protamine, Pro) hybridization. Supramolecular interactions between the crown ring and the guanidinium group in Pro play an important role in the ONPs–Pro hybrid formation. The decorated fluorescent hybrid state is finally used as a nano-probe for sensing heparin via the turn-OFF mechanism. The decoration method is further generalized by recognition of the nucleotides. Herein, we detail the bottom-up approach to the molecular design and development of the different fluorescent states of a useful probe. Most excitingly, this new approach is very general and adaptive to facile detection.

 Please wait while we load your content...
Please wait while we load your content...