Phosphorane lifetime and stereo-electronic effects along the alkaline hydrolysis of phosphate esters†
Abstract
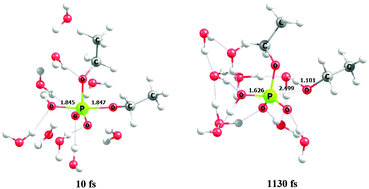
Hybrid quantum mechanical/effective fragment potential (QM/EFP) calculations, in conjunction with the quantum theory of atoms in molecules (QTAIM) and energy decomposition analysis (EDA), were employed to investigate the reaction mechanism and stereo-electronic effects along the alkaline hydrolysis of the monoethyl phosphate dianion (MEP) and the diethylphosphate monoanion (DEP). Reactions proceed through a synchronous bimolecular ANDN mechanism for MEP and a stepwise (AN + DN) mechanism for DEP, with the formation of a phosphorane intermediate, having an overall reaction free energy and barrier of 11.5 and 43.0 kcal mol−1, respectively. In addition, ab initio molecular dynamics simulations were performed to investigate the stability of the phosphorane pentacoordinate intermediate observed in the reaction of the phosphate diester. The phosphorane intermediate has a lifetime of ∼1 ps after which it decomposes into the corresponding alcohol and phosphate monoester dianion. Electrostatics governs the interaction between the nucleophile and the phosphate ester. However, some degree of covalence in the interaction starts to appear at distances shorter than 2.45 Å for MEP and 2.63 Å for DEP. For the monoester, the electrostatic repulsive terms are the dominant contributions for the formation of the transition state. On the other hand, for the phosphate diester, the formation of the P–OH bond is dominated by associative terms of electrostatic nature.

 Please wait while we load your content...
Please wait while we load your content...