Understanding the tautomerism in azacalixphyrins†
Abstract
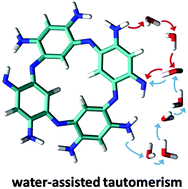
Understanding the chemical nature and spectroscopic signatures of a new class of organic molecules remains a strong challenge. Azacalixphyrin, the first member of a family of strongly aromatic macrocycles absorbing in the near infrared domain, can exist in several tautomeric forms. Here, we use DFT calculations and NMR measurements to propose the first in-depth investigation of proton exchanges occurring in two forms of azacalixphyrins (non-protonated and protonated). Our results reveal, on the one hand, a very effective solvent-assisted tautomerism in the non-protonated form whereas the intramolecular proton transfer is less probable, and, on the other hand, the presence of a mixture of almost isoenergetic tautomers differing in both their aromaticity and absorption profiles. This clearly indicates that smartly-designed chemical substitutions could alter the relative weights of the different tautomers, and consequently tune the optical signatures of these new macrocycles in a versatile and efficient way. For the protonated form, rotations of the NH2 groups take place rather than the chemical exchange.

 Please wait while we load your content...
Please wait while we load your content...