The adsorption and activation of NO on silver clusters with sizes up to one nanometer: interactions dominated by electron transfer from silver to NO†
Abstract
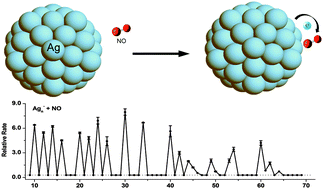
The adsorption and activation of NO on microsilver species provide the foundation to understand the mechanism of NO removing reactions on silver based catalysts. However, the diversiform of the geometrical structures and electronic properties of microsilver species in condensed phases has posed considerable challenges for exploring these interactions. We study the reactions of NO with bare silver clusters Agn± (7–69) in the gas phase using a continuous flow reactor running at low temperatures. Evidence for NO unit adsorption, the formation of (NO)2 and the reduction of NO is observed on different cluster sizes. The kinetic rates of initial NO unit adsorption are closely related to silver clusters' global electronic properties. The low electron binding energy and the unpaired electron of a silver cluster favor the adsorption and activation of NO. In particular, the clusters with one less electron than those of closing electron shells are generally inert and the sizes having one more electron outside these shells are generally quite reactive. These observations depict a general figure of interactions between NO and microsilver species, in which electron transfer from silver to NO dominates.

 Please wait while we load your content...
Please wait while we load your content...