Investigation of trimethylacetic acid adsorption on stoichiometric and oxygen-deficient CeO2(111) surfaces†
Abstract
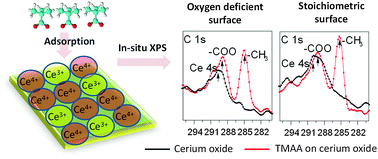
We studied the interactions between the carboxylate anchoring group from trimethylacetic acid (TMAA) and CeO2(111) surfaces as a function of oxygen stoichiometry using in situ X-ray photoelectron spectroscopy (XPS). The stoichiometric CeO2(111) surface was obtained by annealing the thin film under 2.0 × 10−5 Torr of oxygen at ∼550 °C for 30 min. In order to reduce the CeO2(111) surface, the thin film was annealed under ∼5.0 × 10−10 Torr vacuum conditions at 550 °C, 650 °C, 750 °C and 850 °C for 30 min to progressively increase the oxygen defect concentration on the surface. The saturated TMAA coverage on the CeO2(111) surface determined from XPS elemental composition is found to increase with increasing oxygen defect concentration. This is attributed to the increase of under-coordinated cerium sites on the surface with the increase in the oxygen defect concentrations. XPS results were in agreement with periodic density functional theory (DFT) calculations and indicate a stronger binding between the carboxylate group from TMAA and the oxygen deficient CeO2−δ(111) surface through dissociative adsorption.

 Please wait while we load your content...
Please wait while we load your content...