Volume profile of α-chymotrypsin during adsorption and enzymatic reaction on a poly(acrylic acid) brush
Abstract
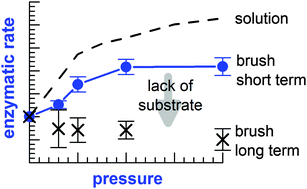
Poly(acrylic acid) (PAA) brushes are known to provide a native-like environment for proteins. In this study, we explore this biocompatibility under high pressure conditions. Using α-chymotrypsin (α-CT) as a model enzyme, we report on the pressure dependencies of the enzymatic activity and the neutron scattering length density profile, when this enzyme is adsorbed on a PAA brush. From high pressure total internal reflection fluorescence spectroscopy, an increasing enzymatic activity has been observed up to 1000 bar, but a rather pressure independent enzymatic activity at higher pressures up to 2000 bar. This finding suggests a non-constant activation volume of α-CT on the PAA brush that is negative below 1000 bar. Thus, the compact nature of the transition state of α-CT is largely preserved upon adsorption. We have also performed high pressure neutron reflectivity experiments to determine the spatial distribution of α-CT inside the PAA brush. Apparently, the enzyme is strongly binding to the PAA chains with 2.3 mg m−2 of adsorbed enzyme that is reduced to about 1.7 mg m−2 at 1000–2000 bar. This change of adsorbed mass is consistent with a positive volume change of adsorption, which is probably reflecting electrostriction upon protein–PAA interaction. Thus, the performed high pressure experiments provide new insights into the volume profile of α-CT during adsorption and enzymatic activity on the PAA brush. They also demonstrate that the biocompatible properties of a PAA brush can even be enhanced by pressure.

 Please wait while we load your content...
Please wait while we load your content...