Transfer of complexed and dissociated ionic species at soft interfaces: a voltammetric study of chemical kinetic and diffusional effects
Abstract
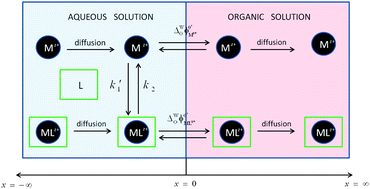
A new transfer mechanism is considered in which two different ionic species of the same charge can be transferred across a soft interface while they interconvert with each other in the original phase through a homogeneous chemical reaction: the aqueous complexation–dissociation coupled to transfer (ACDT) mechanism. This can correspond to a free ion in aqueous solution in the presence of a neutral ligand that complexes it leading to a species that can be more or less lipophilic than the free ion. As a result, the transfer to the organic phase can be facilitated or hindered by the aqueous-phase chemical reaction. Rigorous and approximate explicit analytical solutions are derived for the study of the above mechanism via normal pulse voltammetry, derivative voltammetry and chronoamperometry at macrointerfaces. The solutions enable us to examine the process whatever the species' lipophilicity and diffusivity in each medium and the kinetics and thermodynamics of the chemical reaction in solution. Moreover, when the chemical reaction is at equilibrium, explicit expressions for cyclic voltammetry and square wave voltammetry are obtained. With this set of equations, the influence of different physicochemical phenomena on the voltammetric response is studied as well as the most suitable strategies to characterize them.

 Please wait while we load your content...
Please wait while we load your content...