Flexible additive free H2V3O8 nanowire membrane as cathode for sodium ion batteries†
Abstract
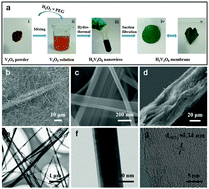
Sodium ion batteries (SIBs) have emerged as a potential candidate to succeed lithium ion batteries (LIBs), because of the abundant sodium resources on earth. Layered vanadium oxides are regarded as the promising candidates for SIBs because of their large interlayer spacing, high theoretical specific capacity, abundant sources and low cost. In this paper, a vanadium oxide hydrate (H2V3O8) nanowire membrane is presented as a flexible cathode for SIBs without addition of any other additives (binders or conductive compounds). Such a freestanding flexible membrane exhibits a high specific capacity of 168 mA h g−1 at 10 mA g−1, and its high capacity is maintained well after 100 cycles. It is found that the capacitive charge storage accounts for a relatively large proportion of the total capacity, whereas the crystal structure of H2V3O8 is highly reversible during the sodiation/desodiation processes. This research demonstrates that the H2V3O8 nanowire is an exceptional candidate for SIBs.


 Please wait while we load your content...
Please wait while we load your content...