Photocatalytic chemistry of methanol on rutile TiO2(011)-(2 × 1)†
Abstract
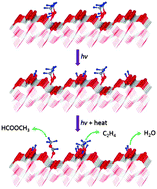
Photocatalytic chemistry of methanol on the reconstructed rutile TiO2(011)-(2 × 1) surface upon 266 nm and 400 nm light excitation has been investigated quantitatively using the post-irradiation temperature-programmed desorption (TPD) method. Photochemical products such as formaldehyde, methyl formate and water, which result from the recombination of surface bridging hydroxyls through the abstraction of lattice oxygen atoms, have been identified under both 266 nm and 400 nm light irradiation. However, ethylene is detected only under 266 nm light irradiation. Through an analogy experiment, ethylene production is attributed to the photochemistry and the following thermochemistry of formaldehyde. The absence of the ethylene signal under 400 nm light is consistent with the significantly lower conversion at this wavelength compared with 266 nm. The photocatalytic reaction rate of methanol is also wavelength dependent. Possible reasons for the photon energy dependent phenomena have been discussed. This work not only provides a detailed characterization of the photochemistry of methanol on the rutile TiO2(011)-(2 × 1) surface, but also indicates the importance of photon energy in the photochemistry on TiO2 surfaces.

 Please wait while we load your content...
Please wait while we load your content...