Pair-eigenstates and mutual alignment of coupled molecular rotors in a magnetic field
Abstract
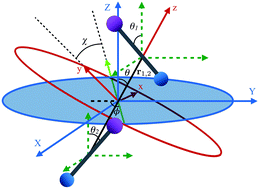
We examine the rotational states of a pair of polar 2Σ molecules subject to a uniform magnetic field. The electric dipole–dipole interaction between the molecules creates entangled pair-eigenstates of two types. In one type, the Zeeman interaction between the inherently paramagnetic molecules and the magnetic field destroys the entanglement of the pair-eigenstates, whereas in the other type it does not. The pair-eigenstates exhibit numerous intersections, which become avoided for pair-eigenstates comprised of individual states that meet the selection rules ΔJi = 0, ± 1, ΔNi = 2n (n = 0, ±1, ±2,…), and ΔMi = 0, ± 1 imposed by the electric dipole–dipole operator. Here Ji, Ni and Mi are the total, rotational and projection angular momentum quantum numbers of molecules i = 1, 2 in the absence of the electric dipole–dipole interaction. We evaluate the mutual alignment of the pair-eigenstates and find it to be independent of the magnetic field, except for states that undergo avoided crossings, in which case the alignment of the interacting states is interchanged at the magnetic field corresponding to the crossing point. We present an analytic model which provides ready estimates of the pairwise alignment cosine that characterises the mutual alignment of the pair of coupled rotors.

 Please wait while we load your content...
Please wait while we load your content...