A density functional theory study of arsenic immobilization by the Al(iii)-modified zeolite clinoptilolite
Abstract
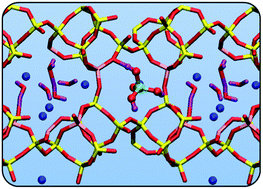
We present density functional theory calculations of the adsorption of arsenic acid (AsO(OH)3) and arsenous acid (As(OH)3) on the Al(III)-modified natural zeolite clinoptilolite under anhydrous and hydrated conditions. From our calculated adsorption energies, we show that adsorption of both arsenic species is favorable (associative and exothermic) under anhydrous conditions. When the zeolite is hydrated, adsorption is less favourable, with the water molecules causing dissociation of the arsenic complexes, although exothermic adsorption is still observed for some sites. The strength of interaction of the arsenic complexes is shown to depend sensitively on the Si/Al ratio in the Al(III)-modified clinoptilolite, which decreases as the Si/Al ratio increases. The calculated large adsorption energies indicate the potential of Al(III)-modified clinoptilolite for arsenic immobilization.

 Please wait while we load your content...
Please wait while we load your content...