Secondary phases and their influence on the composition of the kesterite phase in CZTS and CZTSe thin films†
Abstract
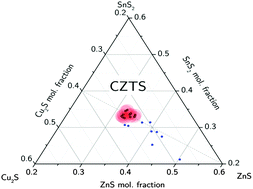
Secondary phases zinc sulfide/selenide and copper sulfide in Cu2ZnSnS4 (CZTS) and Cu2ZnSnSe4 (CZTSe) thin film samples are investigated by X-ray absorption near edge structure (XANES) analysis at the chalcogen K-edges. Because of the formation of secondary phases the composition of the kesterite phase can deviate significantly from the total sample composition. For a large set of non-stoichiometric samples we find that the cation ratios of the kesterite phase never exceed Zn/Sn = 1 even for Zn-rich CZTS and CZTSe, with all excess Zn being contained in secondary phases. For CZTS the cation ratios are found to be additionally constrained by Cu/Sn ≤ 2, which means that Cu-excess always leads to the formation of CuxS secondary phases. These results give clear bounds on the Cu-rich and Zn-rich sides of the single phase region in polycrystalline CZTS/Se thin films.

 Please wait while we load your content...
Please wait while we load your content...