Investigation of the structural preference and flexibility of the loop residues in amyloid fibrils of the HET-s prion†
Abstract
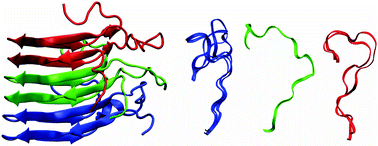
The structural variability of a 16-residue loop (residues 246–261) which is in part disordered and connects two layers of the β-solenoid formed by the prion-form of HET-s and its prion domain HET-s(218–289) is investigated using molecular dynamics computer simulation. A system of three HET-s(218–289) molecules in a β-sheet structure as in the fibril is simulated in aqueous solution. The trajectory structures appear to be consistent with the Cα chemical shift data obtained. In order to delineate the influence of the β-sheet core of the fibril upon the structural variability of the loop, the latter is also simulated without the β-sheet core, but with its N- and C-terminal residues restrained at their positions in the fibril. The analysis of the trajectories shows that the structural variability of the loop is restricted by the β-sheet core, least at its N-terminal end and most in the middle of the trimer.
- This article is part of the themed collection: Exploring the conformational heterogeneity of biomolecules: theory and experiments

 Please wait while we load your content...
Please wait while we load your content...