Dissociative ionization of the 1-propanol dimer in a supersonic expansion under tunable synchrotron VUV radiation
Abstract
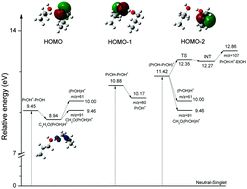
Photoionization and dissociation of the 1-propanol dimer and subsequent fragmentations have been investigated by synchrotron vacuum ultraviolet (VUV) photoionization mass spectrometry and theoretical calculations. Besides the protonated monomer cation (C3H7OH)·H+ (m/z = 61) and Cα–Cβ bond cleavage fragment CH2O·(C3H7OH)H+ (m/z = 91), the measured mass spectrum at an incident photon energy of 13 eV suggests a new dissociation channel resulting in the formation of the (C3H7OH)·H+·(C2H5OH) (m/z = 107) fragment. The appearance energies of the fragments (C3H7OH)·H+, CH2O·(C3H7OH)H+ and (C3H7OH)·H+·(C2H5OH) are measured at 10.05 ± 0.05 eV, 9.48 ± 0.05 eV, and 12.8 ± 0.1 eV, respectively, by scanning photoionization efficiency (PIE) spectra. The 1-propanol ion fragments as a function of VUV photon energy were interpreted with the aid of theoretical calculations. In addition to O–H and Cα–Cβ bond cleavage, a new dissociation channel related to Cβ–Cγ bond cleavage opens. In this channel, molecular rearrangement (proton transfer and hydrogen transfer after surmounting an energy barrier) gives rise to the generated complex, which then dissociates to produce the mixed propanol/ethanol proton bound cation (C3H7OH)·H+·(C2H5OH). This new dissociation channel has not been reported in previous studies of ethanol and acetic acid dimers. The photoionization and dissociation processes of the 1-propanol dimer are described in the photon energy range of 9–15 eV.

 Please wait while we load your content...
Please wait while we load your content...