Theoretical study on the surface stabilities, electronic structures and water adsorption behavior of the Ta3N5(110) surface†
Abstract
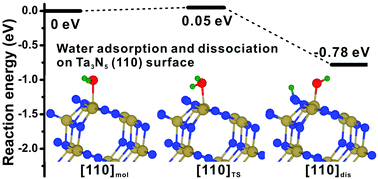
A recent experiment revealed that the Ta3N5 semiconductor with orientation along the (110) surface exhibited improved photoelectrochemical activities, but the role of the (110) surface in the improved photoelectrochemical activity remains unclear. In this study, density functional theory calculations were performed to investigate the surface stabilities, surface electronic structures and water splitting behavior of the Ta3N5(110) surface with and without oxygen impurities. The results showed that, on the clean and oxygen impurity containing (110) surfaces, the energy barriers of water splitting were as low as 0.05 and 0.06 eV, respectively, suggesting that the Ta3N5(110) surface is a promising candidate for water splitting. The lower energy barriers of water splitting on the Ta3N5(110) surface may be ascribed to the easy migration of the H atom from the surface Ta atom to the nearby N atom. Furthermore, the surface energies and surface electronic structures revealed that the Ta3N5(110) surface contained less oxygen impurities, which is in accordance with the experimental observations.

 Please wait while we load your content...
Please wait while we load your content...